
CYGNUS® Matrix is a complete dehydrated placental allograft that contains all native tissue layers (amnion with spongy layer and chorion).1 It provides a protective barrier to support the prevention of adhesions.1,2
As a multi-layered placental allograft, CYGNUS Matrix retains the extracellular matrix (ECM) scaffold, collagen, hyaluronic acid, growth factors, and cytokines.3-5 It is ideal for a variety of surgical procedures and is shelf-stable and convenient to use.1
CYGNUS® Matrix contains all native tissue layers that are never delaminated4,5
CYGNUS® Matrix Layers

Native Placental Tissue Layers

By retaining all native layers, CYGNUS Matrix is up to 4X thicker than the single amnion layer6
CYGNUS® Matrix provides an effective physical barrier and ECM scaffold that helps maintain separate tissue planes for surgical procedures1,2
ECM Proteins
(Hyaluronic Acid & Collagen)
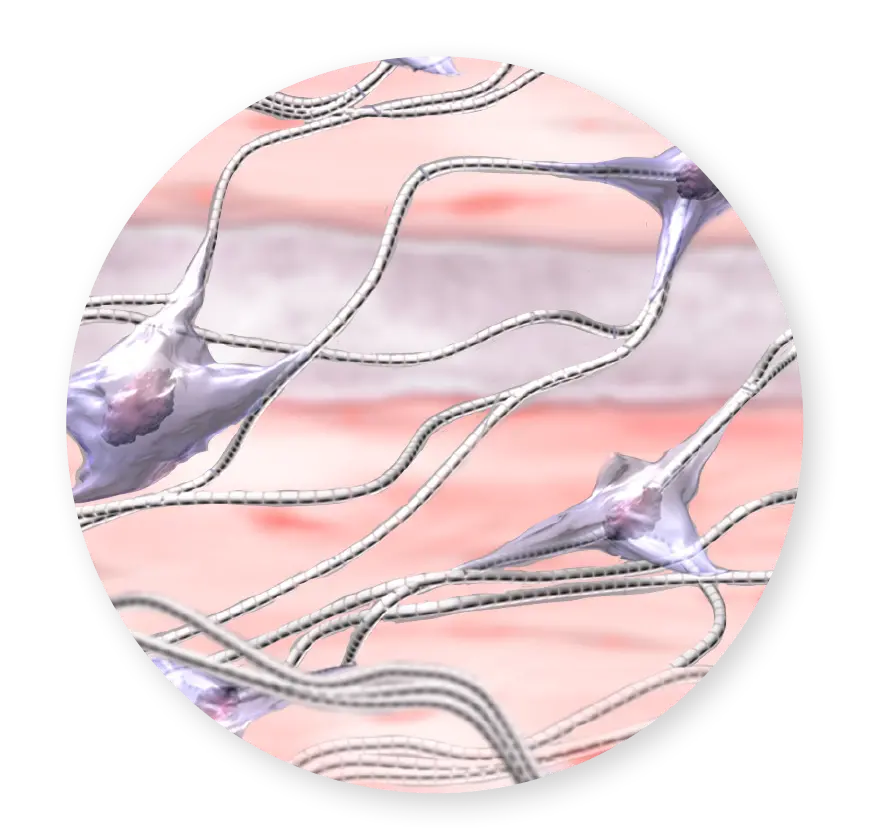
Growth Factors & Cytokines
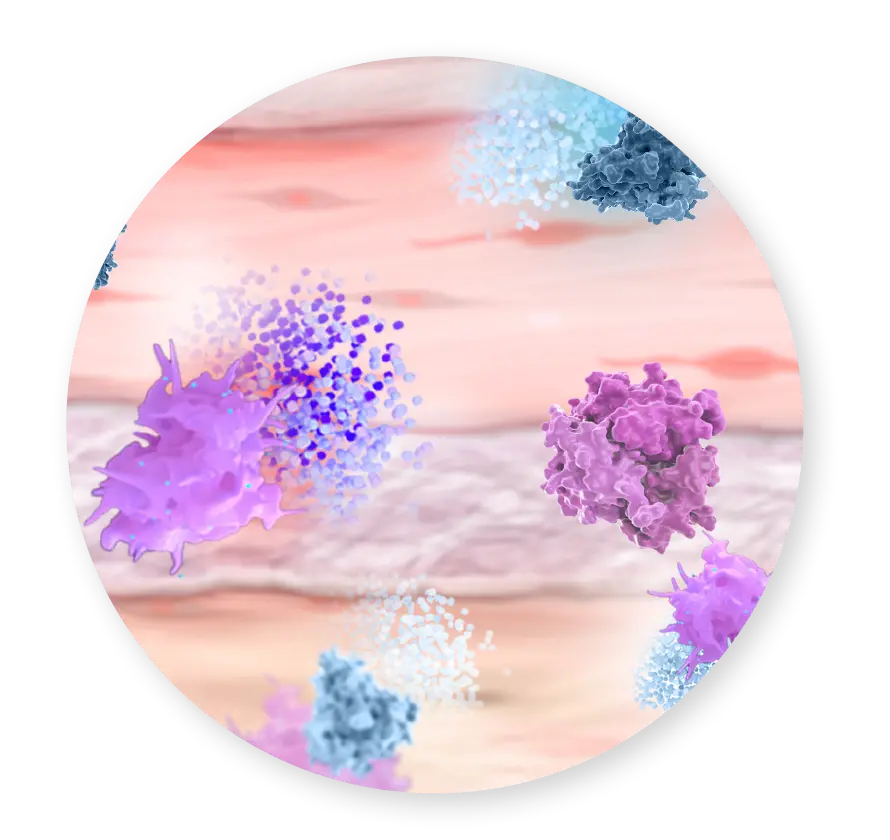
TESTING HAS CONFIRMED THAT CYGNUS MATRIX RETAINS NATIVE COMPONENTS OF PLACENTAL TISSUE3-5
CYGNUS® Matrix provides barrier protection for a variety of surgical procedures,1,2 such as:
Ready-to-use, ambient temperature storage (2°C to 30°C)
No prep required, hydrates in situ
E-Beam sterilized for sterility assurance level (SAL) of 10-6
5-year shelf life
| CYGNUS® Matrix Placental Allograft | |||||
|---|---|---|---|---|---|
| PRODUCT NUMBER | PRODUCT DESCRIPTION | TOTAL SIZE (CM2) | BILLABLE UNITS | HCPCS CODE | UPC |
| 585-014 | CYGNUS Matrix 1.5 cm disc | 2 | 2 | Q4199 | 850023053961 |
| 585-009 | CYGNUS Matrix 2x3 cm | 6 | 6 | Q4199 | 850023053015 |
| 585-010 | CYGNUS Matrix 4x4 cm | 16 | 16 | Q4199 | 850023053039 |
| 585-011 | CYGNUS Matrix 4x6 cm | 24 | 24 | Q4199 | 850023053046 |
| 585-012 | CYGNUS Matrix 6x6 cm | 36 | 36 | Q4199 | 850023053947 |
| 585-013 | CYGNUS Matrix 6x7 cm | 42 | 42 | Q4199 | 850023053954 |
Epithelial layer side up

Please refer to the CYGNUS Matrix instructions for use for usage and safety information.