VIA Matrix is a multi-layer placental membrane allograft, which includes the amnion, intermediate/spongy and chorion layers
Retains naturally occurring structural components, specifically, collagen and hyaluronic acid.1,2
Layers are never delaminated; structural integrity of the amnion, intermediate/spongy, and chorion is maintained.
VIA Matrix is composed of an extracellular matrix (ECM) scaffold and provides a protective barrier to support the body's ability to repair underlying damaged tissue, such as acute and chronic wounds1-4
ECM Proteins
Growth Factors & Cytokines
TESTING HAS CONFIRMED THAT VIA MATRIX RETAINS NATIVE COMPONENTS OF PLACENTAL TISSUE3,4
VIA Matrix may be used to support healing in a wide range of partial- and full-thickness acute and chronic wounds1
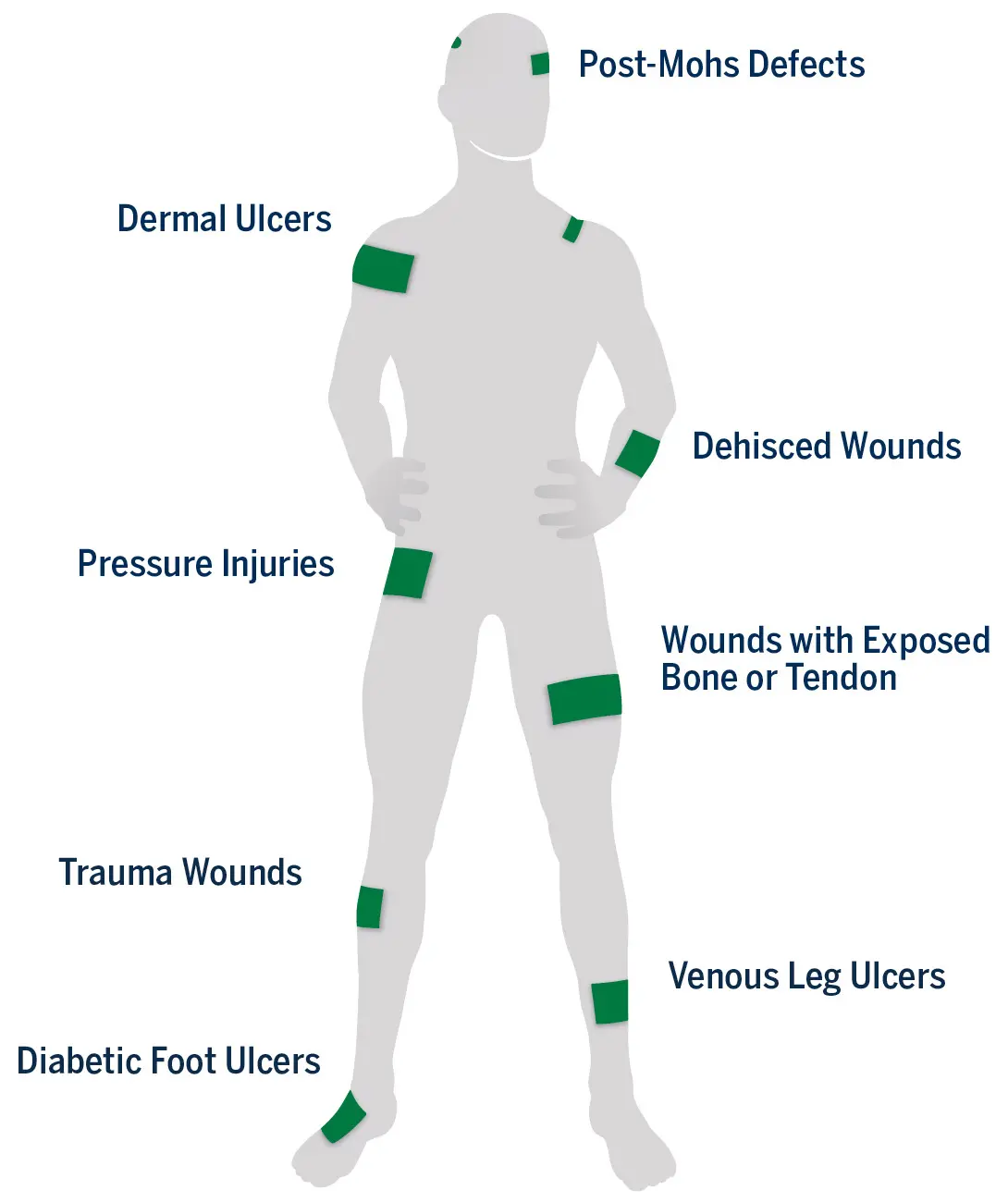
Ready-to-use, ambient temperature storage (2°C to 30°C)
No prep required, hydrates in situ
E-Beam sterilized for sterility assurance level (SAL) of 10-6
5-year shelf life
| VIA Matrix Placental Allograft | |||||
|---|---|---|---|---|---|
| PRODUCT NUMBER | PRODUCT DESCRIPTION | TOTAL SIZE (cm2) | BILLABLE UNITS | HCPCS CODE | UPC NUMBER |
| 585-005 | VIA Matrix 2x3 cm | 6 | 6 | Q4309 | 850023053855 |
| 585-006 | VIA Matrix 4x4 cm | 16 | 16 | Q4309 | 850023053848 |
| 585-007 | VIA Matrix 4x6 cm | 24 | 24 | Q4309 | 850023053862 |
| 585-008 | VIA Matrix 10x11 cm | 110 | 110 | Q4309 | 850023053923 |

A comprehensive program that provides your practice with the highest level of customer service and reimbursement support including:
Please refer to the VIA Matrix instructions for use for usage and safety information.