As a dual-layered, dehydrated amniotic membrane, CYGNUS® Dual supports an optimal environment for healing wounds while offering streamlined product application and practice logistics.1-4
With two layers of human amnion allograft, CYGNUS Dual is 2x thicker than single-layered amniotic allografts and is approximately 200 µm (0.2mm) thick.5

Image of CYGNUS Dual dehydrated amnion/amnion
The epithelial layers face outward allowing for omni-directional application. Because either side of the product may be applied to the wound bed, CYGNUS Dual helps streamline procedures.1
CYGNUS Dual is composed of an extracellular matrix (ECM) scaffold and provides a protective barrier to support the body's ability to repair underlying damaged tissue, such as acute and chronic wounds1-4
ECM Proteins
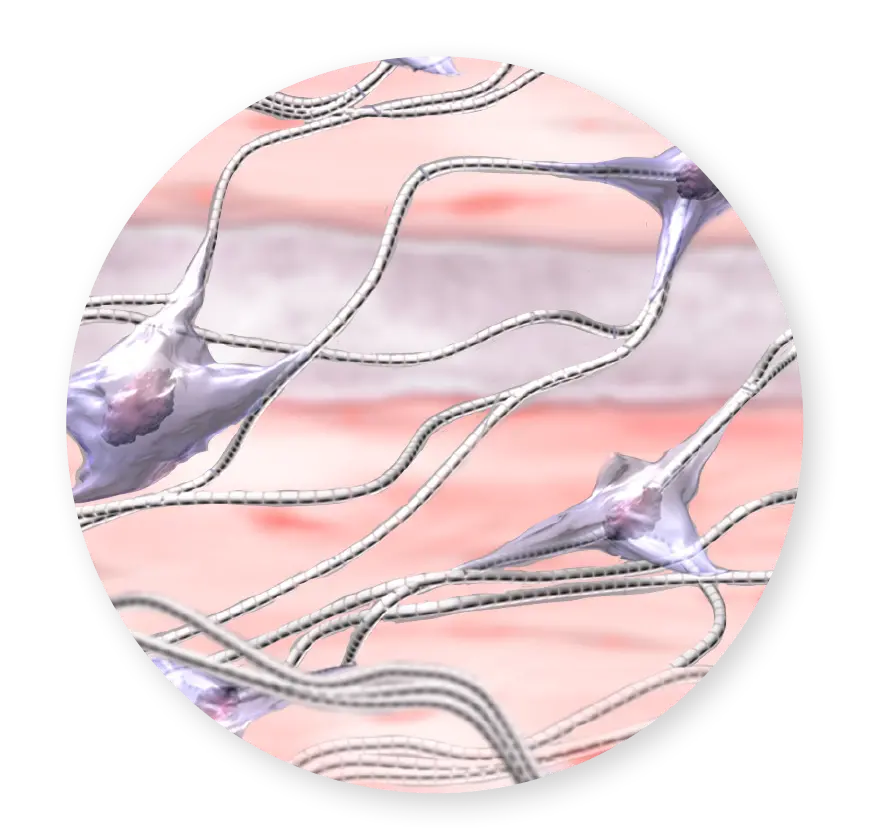
Growth Factors & Cytokines
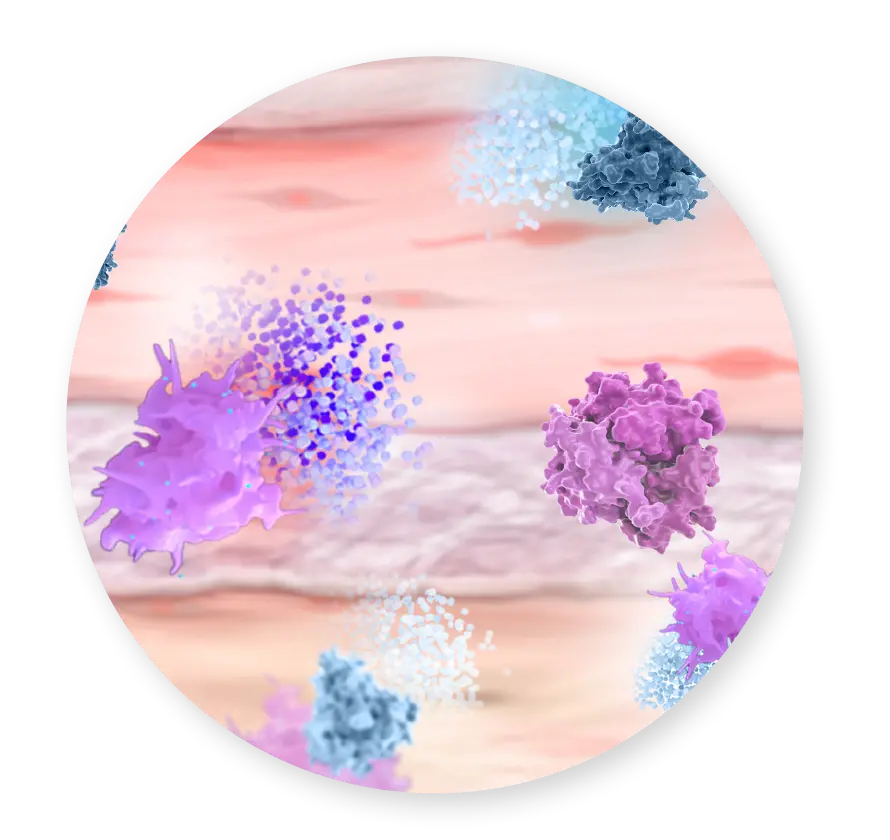
TESTING HAS CONFIRMED THAT CYGNUS DUAL RETAINS NATIVE COMPONENTS OF AMNIOTIC TISSUE3,4
CYGNUS Dual may be used to support healing in a wide range of partial- and full-thickness acute and chronic wounds1
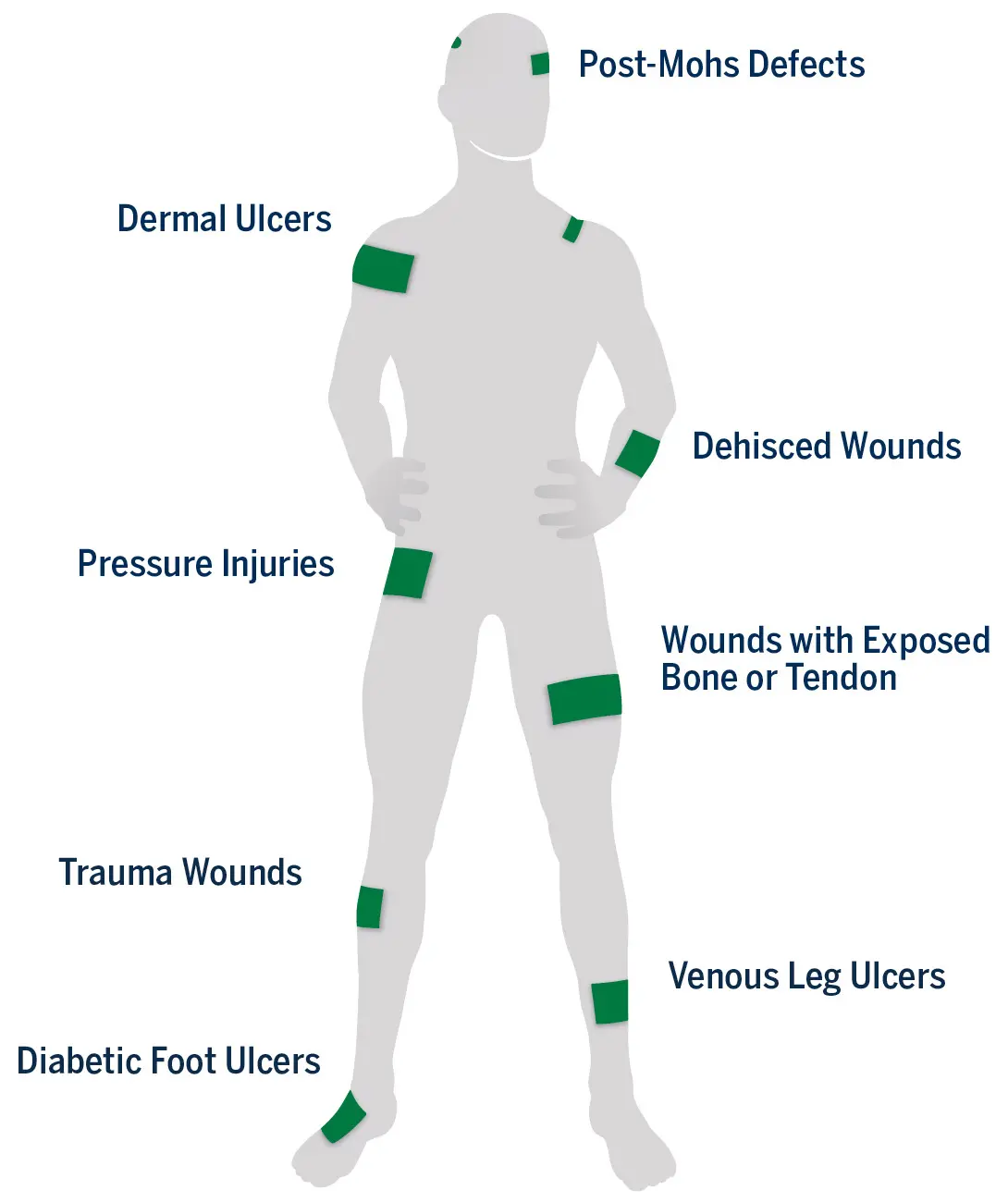
Ready-to-use, ambient temperature storage (2°C to 30°C)
No prep required, hydrates in situ
E-Beam sterilized for sterility assurance level (SAL) of 10-6
5-year shelf life
| CYGNUS DUAL Amnion Allograft | |||||
|---|---|---|---|---|---|
| PRODUCT NUMBER | PRODUCT DESCRIPTION | TOTAL SIZE (cm2) | BILLABLE UNITS | HCPCS CODE | UPC NUMBER |
| 585-001 | CYGNUS DUAL 2x3 cm | 6 | 6 | Q4282 | 850023053800 |
| 585-002 | CYGNUS DUAL 4x4 cm | 16 | 16 | Q4282 | 850023053817 |
| 585-003 | CYGNUS DUAL 4x6 cm | 24 | 24 | Q4282 | 850023053824 |
| 585-004 | CYGNUS DUAL 7x15 cm | 105 | 105 | Q4282 | 850023053831 |

A comprehensive program that provides your practice with the highest level of customer service and reimbursement support including:
Please refer to the CYGNUS Dual instructions for use for usage and safety information.